Diagnosis
Objectives
By the end of this session trainees are expected to:
- Know the case definition of visceral leishmaniasis
- Recognize the different lab diagnostics, their strengths and limitations
- Understand the diagnostic algorithm
Recommended facilitation strategy
- Facilitate reading of the manual
- Provide different case scenarios on the use of the diagnostic algorithm
- Select from the cases at the end of this manual and initiate group work
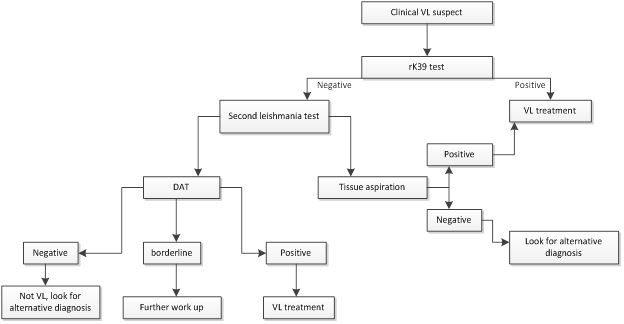
It is important to suspect and establish the diagnosis of VL as early as possible so that the appropriate treatment is initiated. Unless treated, VL is universally fatal. Untreated cases may also act as reservoirs and play role in the ongoing transmission in the community. Diagnostic methods for VL are clinical, serology, parasitological and molecular.
Clinical diagnosis: This is based on the signs and symptoms. VL case is defined as a person from endemic area or with travel history presenting with more than two weeks of fever, weight loss and splenomegaly. The probability of VL among suspects based on the above clinical case definition is 40-60%. Diagnosing VL based on clinical presentation alone lacks specificity and requires definitive test. Thus additional lab investigations are used.
Parasitological: Confirmatory diagnosis of VL relies on microscopic visualization of the amastigote forms of the parasite on Giemsa stained tissue aspirations (spleen, bone marrow, lymph nodes). While splenic aspiration has the highest diagnostic yield, 95%; it is potentially associated with serious bleeding. One has to check for contraindications before the procedure, and this procedure can be done only at a facility where it is possible to manage the complications. Blood transfusion and major surgical services are needed as a prerequisite in a health facility to do splenic aspiration. A 21-gauge needle and a 5ml syringe are recommended and it should be done by experienced physician.
The contraindications for splenic aspiration are:
- Platelet count less than 40,000/µl
- The presence of clinical bleeding tendency irrespective of the platelet count
- Ascites
- Advanced pregnancy
- Spleen size less than 3cm below the costal margin
- Non-cooperative patients for the procedure due to understanding problems or children
- Severe anemia with hemoglobin level <5gm/dl
- Deep jaundice
Bone marrow and lymph node are the other alternative tissues that can be aspirated but are less diagnostic than the spleen (53 – 86% and 53-65% respectively).
Another means of parasitological diagnosis is culturing the tissue aspirate on MacNeal-Nicolle-Novy (NNN) or RPMI media. Culture improves the diagnostic yield but it takes about five days to see the growth.
Demonstration of parasite from peripheral blood, buffy coat is also possible but with low yield (<30%). It is higher among HIV coinfected patients and those with high tissue parasite load (>+4). Here it may reach upto 50%.
Serology tests: There are antibody and antigen detection methods.
Antibody detection – there are several methods of antibody detection techniques for leishmania. These include:
- Enzyme linked immunosorbent assay (ELISA)
- Immunofluorescent antibody test (IFAT)
- Freeze dried direct agglutination test (FD-DAT)
- Aqueous antigen direct agglutination test (AQ-DAT)
- Fast agglutination screening test (FAST)
- Indirect hemagglutination test (IHA)
- rK39 immunochromatographic strip test
- rK39 ELISA
The performance of the kits was found varying in sensitivity and specificity from 70% to 100%. Antibody detection has two major limitations: 1. They cannot distinguish infection from disease. As a result most people from endemic areas test positive and can be over diagnosed as visceral leishmaniasis. 2. They cannot be used for treatment monitoring. The titer remains high even after effective treatment. Thus, they cannot help to diagnose relapse. rK39 ICT and DAT are widely studied and used in several set ups.
rK39 immunochromatographic test (ICT) – This is a recombinant K39 antigen based dipstick. K39 is a 39aminoacid repeat that is part of a kinesin protein of L chagasi that is also conserved with L donovani complex. It is commercially available in different formats: DiaMed-IT Leish, InBios, Kala-azar detect. The performances of the kits differ with the brand and geographic area. Generally, the performance of rK39 RDT was found consistently lower in East Africa region as compared to the Indian Subcontinent. A meta-analysis has shown that the pooled sensitivity and specificity of rK39 ICT is 85% and 91% respectively in East Africa region as compared to 97% and 90% respectively in Indian Subcontinent.
Due to the easiness of rK39 ICT for use in field setting, its rapid result, and its cheaper cost, it is widely used and recommended in the diagnostic algorithms. However, rK39 should be used only on those patients who fulfill the clinical case definition of VL (fever >2 weeks, splenomegaly, weight loss and from VL endemic area or with travel history to VL endemic area). While a positive rK39 test result in a clinical suspect strongly favors VL (positive predictive value 86-93%), a negative result does not strongly exclude VL (negative predictive value 81-90%). Thus, a clinically suspected patient with rK39 test negative needs further evaluation and tests for VL. If the algorithm is not respected, i.e. if rK39 test is performed in a patient who does not fulfill the clinical case definition, then the predictive value of the test may even deteriorate.
Direct Agglutination Test (DAT) – This is a semi-quantitative test based on freeze dried or liquid antigens from the whole parasite. While the liquid antigen requires refrigeration, freeze dried does not. The cut off value of 1/3200 is often used as indicator of disease. Alternatively, <1/1600 negative; 1/1600-1/12800 borderline and >1/12800 positive are also used. Depending on the local sensitivity and specificity this cut off may vary. Physicians may decide about the patients in borderline ranges based on additional clinical features and exclusion of the differentials.
Antigen detection methods – Ideally, antigen detection would have been more specific and can help for treatment monitoring. However, this is not yet well developed. KAtex, a heat stable, low molecular weight carbohydrate antigen excreted in the urine of VL patients, is a test kit existing so far but with low diagnostic performance. Its sensitivity is 48%-87%. Its use for treatment monitoring is also not yet fully investigated and standardized.
Molecular diagnostics
Polymerase chain reaction (PCR) based parasite DNA detection from peripheral blood or tissue aspirates has high sensitivity and specificity (>95%). Real-time PCR enables quantification of parasite burden and can be used for detection of active disease and follow up. This is especially important for HIV patients who remain with chronic infection. It allows non-invasive monitoring of patients. However, PCR needs high technology, expertise and is costly. It is a diagnostic and screening test for VL in developed countries. There is no point of care test or a molecular diagnostic kit easy to use in field settings to date. In addition, the applicability of PCR in highly endemic areas is questionable as asymptomatic individuals from endemic areas become positive and interpretation needs care.
Leishmanin skin test – also called Montenegro test is a compound from promastigote culture. It assesses the cell mediated immunity. Thus, it is negative (no reaction) during active disease. It can help to screen asymptomatic infection in endemic areas and has epidemiologic value than diagnosis.
Procedure: 0.1ml of the compound is injected intradermally and the reaction is read after 48hours by measuring the induration size. Greater than 5mm induration is considered positive test. It becomes positive after 12 months of VL treatment. CL patients can produce positive result.
References:
Boelaert M, et al. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease (review), the Cochrane library 2014, issue 6
Diro E et al. Impact of the use of a rapid diagnostic test for visceral leishmaniasis in clinical practice in Ethiopia: a retrospective study, PlosNTD 9(5), 2015